
Validation of Cleaning Process for Reusable Medical Devices
FDA Guidance for industry and Food and Drug Administration Staff
AAMI TIR 12: 2020
AAMI TIR 30: 2011/(R)2016
ISO 15883-1
ISO 15883-2
ISO 15883-5
ISO 17664: 2017
YY/T 0802-2020
WS/T 367-2012
WS 310.1-2016
WS 310.2-2016
WS 310.3-2016




Manufacturers of reusable medical devices must provide instructions on how to reprocess their devices before next use. Three types of risks are associated with the reuse of a medical device: (a) the risk of disease transmission from one patient to another or from environmental sources to a patient; (b) the risk of inadequate or unacceptable device performance following reprocessing; and (c) the risk of occupational exposure to bloodborne pathogens and other potentially infectious materials. Reprocessing involves several steps, including cleaning, testing for device performance, disinfection, and/or sterilization.
Cleaning a device is the critical first step in reprocessing any device after it has been used on a patient. Failure to remove foreign material (e.g., soil, lubricants, microorganisms, organic and inorganic materials) from both the outside and the inside of the device can interfere with the effectiveness of subsequent disinfection and/or sterilization.
Cleaning validation process:
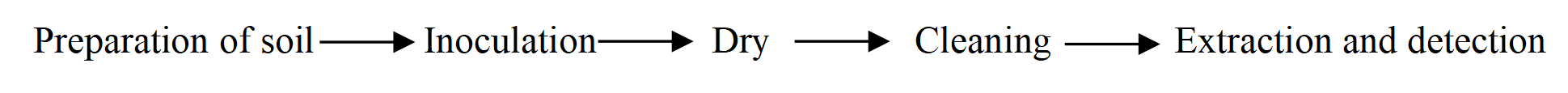
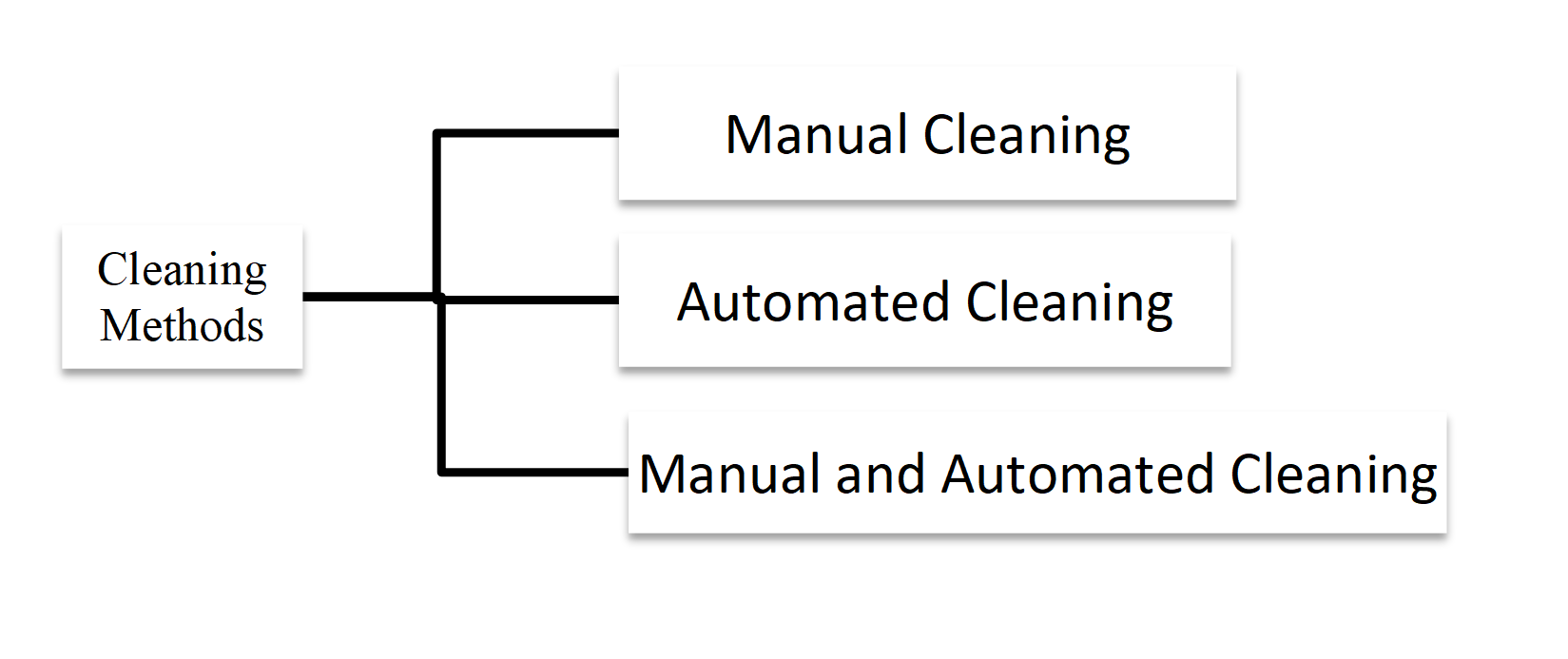
Referred Standards:
Ø FDA Guidance for industry and Food and Drug Administration Staff
Ø AAMI TIR 12: 2020
Ø AAMI TIR 30: 2011/(R)2016
Ø ISO 15883-1
Ø ISO 15883-2
Ø ISO 15883-5
Ø ISO 17664: 2017
Ø YY/T 0802-2020
Ø WS/T 367-2012
Ø WS 310.1-2016
Ø WS 310.2-2016
Ø WS 310.3-2016
Test duration: 4~6 week
Need at least 6 pcs test articles.
Copyright © EPINTEK GROUP
Powerd by PEERHI
EPINTEK GROUP
Tel: +86 21 54736833
Address: 4th Floor, T2 Wanjin Center, Lane 360, Xinlong Road, Minhang District, Shanghai
E-mail: stefanie.sun@epintek.com




We Focus on the Demand for Innovation
